Background
Cell metabolism is a highly regulated process essential for sustaining life and driving evolution. Cellular metabolites and metabolic flux intricately govern post-translational modifications of proteins. In the context of acute graft-versus-host disease (aGVHD), T cells undergo enhanced glycolysis, resulting in increased lactate production. Consequently, lactate may act as a substrate for lysine lactylation (Kla), influencing gene expression by modulating histone biology. This study aims to assess T cell glucose metabolism during aGVHD, investigate the role of histone lactylation, and explore its impact on T cell functionality.
Methods
We conducted a comparative analysis of histone lactylation between aGVHD patients and individuals undergoing allo hematopoietic stem cell transplantation (control). Subsequently, we established a murine bone marrow transplant model and isolated T cells for analysis. Glucose metabolism status was assessed using extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) assays, along with the 2-NBDG glucose uptake assay. To comprehensively investigate histone lactylation in T cells, we employed the cleavage under targets and tagmentation (CUT&Tag) technique to explore its regulatory effects on gene expression. To investigate the impact of lactatylation on aGVHD pathology, we constructed an aGVHD model by applying T cells with a specific deletion of LDHA.
Results
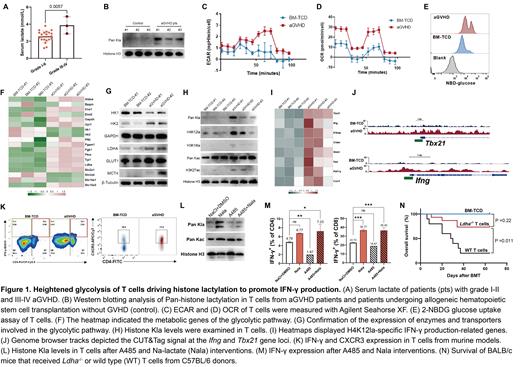
By analyzing the serum lactate at the onset of aGVHD, we found that patients with grade III-IV aGVHD had significantly higher lactate levels compared to those with grade I-II aGVHD ( Figure 1A). In light of this finding, we further examined the histone lactylation levels in T cells from aGVHD patients. The results demonstrated an enhanced histone lactylation in these T cells compared to controls ( Figure 1B).
In the aGVHD mice, the ECAR of T cells exhibited a significant increase ( Figure 1C). Intriguingly, the OCR were also significantly higher in the aGVHD mice compared to the bone marrow T cell-depleted (BM-TCD) mice ( Figure 1D). Additionally, the 2-NBDG revealed a significantly higher fluorescence intensity in aGVHD mice ( Figure 1E). Through analysis of T cell RNA-seq data, we discovered upregulation of most glycolytic enzymes in aGVHD mice similar to human data ( Figure 1F). Notably, LDHA, glucose transporter 1 ( Slc2a1), and monocarboxylate transporter 4 ( Slc16a3) were highly upregulated ( Figure 1G).
The upregulation of LDHA in T cells from aGVHD mice prompted us to investigate the presence of histone lactylation. The results revealed that histone in T cells from aGVHD mice exhibited a higher level of lactylation ( Figure 1H). Because lactylation was particularly prominent at the H4K12 site, we conducted CUT&Tag experiments. We found promoters of key regulatory genes of the Th1 subset, such as Tbx21 and Ifng, along with several genes associated with IFN-γ production, including Stim2, Crtam, Il18rap, Fasl, and Phf11a, Cxcr3 were all lactylated ( Figure 1I). Specifically, the Call Peak analysis confirmed the elevated H4K12la levels on the promoters Tbx21 and Ifng ( Figure 1J). The expression of IFN-γ and CXCR3 in CD4 + and CD8 + T cells of aGVHD mice was significantly higher than in BM-TCD mice ( Figure 1K).
To confirm the role of histone lactylation in IFN-γ generation, we intervened with T cells using the p300 lactylation writer inhibitor (A485). The results demonstrated a significant reduction in histone lactylation upon A485 treatment, which was effectively restored by the addition of neutral lactate sodium (Nala) ( Figure 1L). Notably, A485 treatment led to a decrease in IFN-γ + T cells, while Nala administration resulted in a substantial recovery of IFN-γ expression ( Figure 1M). Additionally, the infusion of Cd4creLdhafl/fl T cells into recipient mice improved the survival of aGVHD mice ( Figure 1N).
Conclusion
This study represents the first global investigation of T cell histone lactylation in aGVHD. Importantly, we have uncovered a novel link between histone lactylation and the regulation of IFN-γ-related gene expression in the context of aGVHD. Considering the heightened glycolysis and the pivotal role of IFN-γ in aGVHD pathology, it is plausible to postulate that T cell-produced lactate induces histone lactylation, thereby promoting the transcription of IFN-γ-related genes and contributing to the development of aGVHD.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal